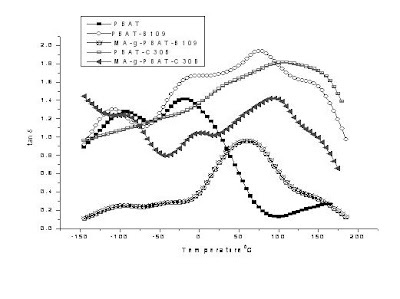
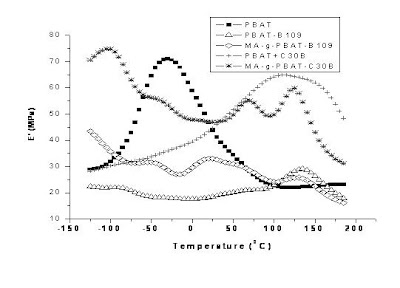
6.2. Thermo Gravimetric analysis (TGA)
The thermal stability of virgin PBAT, PBAT bio-nanocomposite hybrids and MA-g-PBAT bio-nanocomposites are assessed employing TGA. The initial degradation temperature and temperature at 50% degradation and ash content is represented in figure 6 and table 6.
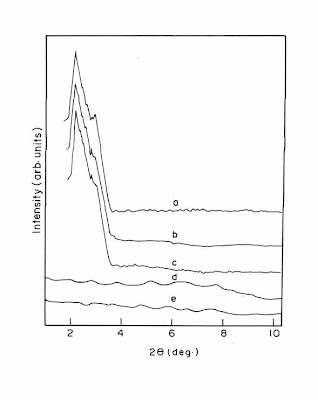
Figure 6: Thermo Gravimetric analysis (TGA) for PBAT and its Bio-nanocomposite
(a). PBAT, (b). PBAT-Na+MMT, (c). PBAT-C20A, (d). PBAT-C30B, (e). PBAT-B109, (f). MA-g-PBAT-C30B, (g). MA-g-PBAT-B109
It is evident that the thermal degradation of virgin PBAT starts at 310.58°C and 100% degradation were noticed around 412°C, whereas incorporation of organically modified nanoclays substantially increases the thermal stability of the biopolymer. PBAT/C30B nanocomposite hybrid exhibits the initial degradation temperature around 322.58°C and final degradation temperature around 469.58°C which is comparatively higher than that of virgin matrix. A similar increase in the initial and final degradation temperature of PBAT/B109 nanocomposite hybrid to 326.23°C and 469.24°C was observed. The phenomenon of increase in thermal stability of the biopolymer matrix with the addition of layered silicates is primarily due to the fact that the nanoclays act as heat barrier, thereby increasing the thermal stability of the system as well as assisting in char formation during thermal decomposition.
The grafted bio-nanocomposite hybrids exhibited a further increase in the degradation temperature. MA-g-PBAT/B109 showed maximum initial and final degradation temperature of 339.59°C and 505.82°C. The bio-nanocomoposite hybrid samples prepared using B109 nanoclay exhibited optimum thermal performance owing to its higher surface area and smaller platelets.
In case of the nanocomposite hybrid a char residue was obtained which indicated improved flammability characteristic in the system.